

Your contact persons
Social issues such as access to medicines for all patients are important for the entire pharmaceutical industry. As far as the safety of these medicines is concerned, CHEPLAPHARM's business model is associated with comparatively low risks due to its long-standing positive track record. We market our diversified portfolio of around 150 products in 145 countries worldwide. As a result, we help health-impaired people with a wide range of indications, including rare diseases. One third of our medicines are on the WHO's "essential medicines list" and are therefore considered essential globally.
Our highest priority: The quality and safety of our products
CHEPLAPHARM supplies pharmaceutical products to millions of patients around the world and fulfils this responsibility sustainably by providing safe and reliable products. For this reason, our company currently employs more than 100 people in its own quality assurance department. Responsibility for quality assurance lies with the Director Quality and CMO at the second management level and with Dr Bianca Juha on the Executive Board. Regular safety audits are a matter of course for us. This pays off: In the 2023 financial year, our recall rate was 0.00%.

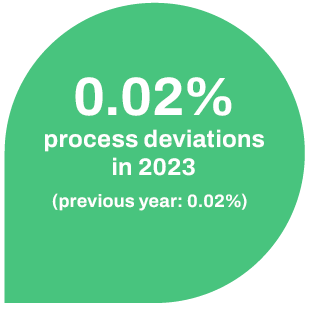
To monitor and control outsourced activities, CHEPLAPHARM also utilises an established quality management system that is anchored in the quality assurance agreements of the contracts with partner companies. For 2023, suppliers, who account for around three quarters of the purchasing volume recorded, stated that they have a certified quality management system in accordance with ISO 9001 or a comparable standard. In 2023, there were 0.02% deviations in our defined quality processes in relation to the number of batches released (0.02% deviations in 2022).
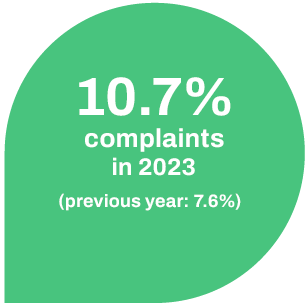
The number of complaints in relation to the total number of approved batches was 10.7% in 2023 (2022: 7.6%) and has therefore increased slightly.


Recruiting, retaining and training talent regardless of gender, origin, religion or other characteristics is fundamental to us. As our organization is constantly growing, we created an independent department for targeted HR and organizational development at the beginning of 2023.
We want to create a family-friendly working environment for our employees. Part-time models, flexible working hours and mobile working are a matter of course for us in order to facilitate the work-life balance. It is also important to us to promote talented and motivated employees and at the same time to retain them. In addition, we prepare our employees specifically for management positions by offering them leadership training. We enable our female employees to participate in a mentoring program for women in business run by the state of Mecklenburg-Western Pomerania. As a result, we currently have a 56% share of women compared to an average of 45% in other pharmaceutical companies in Germany.


As a company with a highly scalable asset-light business model, CHEPLAPHARM has outsourced the manufacturing of its products to a diversified network of more than 125 CMOs and suppliers. Full respect for universal human rights along our entire value chain is highly relevant to us. We condemn all forms of exploitation, especially forced and child labour, and are committed to humane working conditions and fair pay. We demand the same from our suppliers. All of CHEPLAPHARM's suppliers are audited annually for compliance with social standards, which include topics such as occupational safety, diversity, remuneration and many others. Suppliers that have proven to be particularly stable and sustainable are favoured in subsequent projects. When making initial contact with potential new suppliers, we also ensure that they comply with the social standards we require. In addition, location and development-related risks are evaluated and form part of the assessment of our supply chain, which is carried out by our specially sensitised colleagues. CHEPLAPHARM will take appropriate measures if there are any deficits in terms of social standards within the existing supply chain.
Compliance with local laws on labour rights at our suppliers is a matter of course for us. We attach great importance to the implementation of further-reaching employee rights, such as those defined by the general standards of the International Labour Organization (ILO). These standards attach particular importance to the safety of products and employees in the manufacture of pharmaceutical products. We also expect our suppliers to fully respect these standards.
Last but not least, we would like to take this opportunity to refer once again to our central codes of conduct: Our Code of Conduct and our Supplier Code of Conduct summarise the core values of CHEPLAPHARM and define behaviour that complies with the law and guidelines - both documents are publicly accessible on our website for our employees, suppliers and sales partners as well as all other stakeholders and must be complied with. CHEPLAPHARM reserves the right to audit suppliers every three years and - if sufficient evidence is provided - to commission an external audit.